The large-scale genomics study has helped to disentangle the attributes of Lp(a) that drive cardiovascular risk.
Continued progress in lowering the worldwide toll from heart disease may hinge on deeper insights into a stealthy blood fat called lipoprotein(a), or Lp(a). While there is a link between higher levels of Lp(a) and cardiovascular risk, the complexity of this lipid has made it hard to pin down the nature of the risk.
Unlike its molecular cousin and well-known heart disease culprit LDL cholesterol, Lp(a) particles vary greatly in size depending on the genes you inherit. To date, it has been unclear if the danger posed by high Lp(a) is due to the size of the particles you inherit, the concentration of particles in the blood, or a mix of both factors. Resolving this question is vital to determining the best way to measure Lp(a), calculate risk, and identify patients in need of therapy.
The largest-ever genomics study of Lp(a) has provided substantial evidence that the cardiovascular risk from high Lp(a) can be explained by its concentration in the blood. The study was led by scientists at Amgen’s deCODE Genetics subsidiary along with colleagues from the National University Hospital in Iceland. They reached their conclusions based on whole genome data from about 150,000 Icelanders participating in deCODE’s research, including 18,000 with coronary artery disease (CAD) and 9,000 with type 2 diabetes. The results were published in the Journal of the American College of Cardiology (JACC), along with an editorial that discussed the significance of the findings.
“This study from our little island may well turn out to be an important contribution to global health,” said Kári Stefánsson, CEO of deCODE and a senior author on the paper. “We have very effective LDL-lowering drugs, but heart disease remains the biggest killer worldwide. Understanding Lp(a) is key to addressing residual risk.”
“The findings are of particular importance because, if replicated in other studies with more diverse populations, they could help eliminate one of the hurdles of using Lp(a) as a therapeutic target for residual cardiovascular risk in patients with high Lp(a) levels,” said the editorial’s author, Benoit Arsenault, an associate professor in the Department of Medicine at the Université Laval in Quebec, Canada.
Up to double the risk for heart disease observed
Lp(a) consists of an LDL cholesterol-like particle with a second protein, called apolipoprotein(a), or apo(a), coiled around it (see illustration). There are many different gene variants for apo(a) that make longer or shorter versions of the protein.
The deCODE team directly measured Lp(a) blood concentrations in approximately 12,000 study participants, and then used these data to impute the blood levels of the remaining participants. That was possible because Lp(a) levels are driven almost solely by the genes you inherit, and deCODE has developed statistical methods that leverage genealogical data to track genes that travel together among blood relatives.
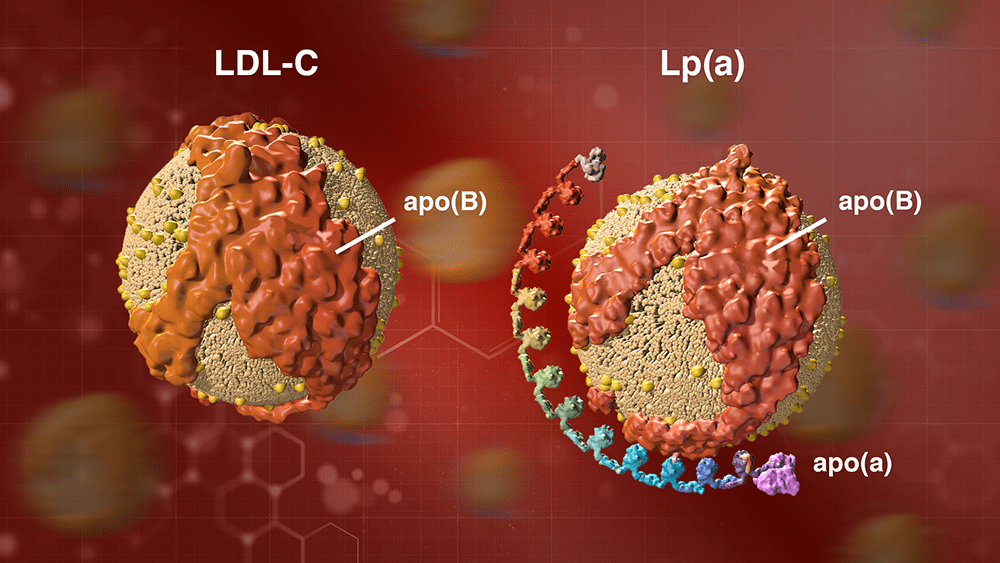
A particle of LDL cholesterol on the left and a particle of Lp(a) on the right. Lp(a) consists of an LDL cholesterol-like particle with a second protein, called apolipoprotein(a), or apo(a), coiled around it. The apo(a) protein can be longer or shorter, depending on the genes you inherit.
Previous studies have suggested that the size of Lp(a) particles helps to determine risk, and that smaller particles are associated with higher risk. In assessing the role of particle size in risk, the deCODE team looked at a fairly common variant of the LPA gene, G4925A, which leads to smaller Lp(a) particles but lower concentrations of this lipid. Despite having smaller particles, individuals who carry the G4925A variant are at no higher risk for heart disease than non-carriers, contributing to the evidence that the number of Lp(a) particles in the blood, not their size, drives risk.
Across the entire population that took part in the study, the median blood concentration of Lp(a) was 14 nanomoles (nM) per liter. Every 50 nM increase in Lp(a) level was associated with a 16 percent increase in the risk for coronary artery disease (CAD). About 7 percent of the study participants had Lp(a) levels over 150 nM, which was predicted to increase CAD risk by 50 percent. About 1 percent of participants had an Lp(a) level above 250 nM, which was predicted to double the risk of CAD.
The study also demonstrated that increased Lp(a) levels correspond with risk for peripheral artery disease, aortic valve stenosis, heart failure, and reduced lifespan. In addition, the study replicated the observation that very low levels of Lp(a)—below 3.5 nM—are associated with increased risk of type 2 diabetes.
Global testing for Lp(a) needed
Stefánsson noted that the impact of elevated Lp(a) levels on heart disease risk “is independent of other risk factors and is 95 percent genetically determined, so you can't reduce it by improving your lifestyle or diet. The clear message is therefore that we need to test for Lp(a) globally to identify those at significantly elevated risk, and speed the development of new therapies aimed at silencing the apo(a) gene. The good news is that our colleagues at Amgen as well as other pharmaceutical companies are already taking such therapies into clinical trials."
“We know that Lp(a) is an independent risk factor for cardiovascular disease, and Amgen is proud of the work we have done and continue to do in pursuing this therapeutic target,” said Dave Reese, Amgen’s executive vice president for Research and Development. “This new study from deCODE will help steer and inform research into the clinical development of molecules targeting Lp(a). It’s a great example of the potential we are just beginning to uncover using genetic insights and validation to power drug development.”
An important next step in this research will be to validate the results in other populations and determine how the contribution of Lp(a) levels to heart disease might vary between populations of different ancestry.
Forward-Looking Statements
This news release contains forward-looking statements that are based on the current expectations and beliefs of Amgen. All statements, other than statements of historical fact, are statements that could be deemed forward-looking statements, including any statements on the outcome, benefits and synergies of collaboration with any other company, including BeiGene, Ltd., or the Otezla® (apremilast) acquisition, including anticipated Otezla sales growth and the timing of non-GAAP EPS accretion, as well as estimates of revenues, operating margins, capital expenditures, cash, other financial metrics, expected legal, arbitration, political, regulatory or clinical results or practices, customer and prescriber patterns or practices, reimbursement activities and outcomes and other such estimates and results. Forward-looking statements involve significant risks and uncertainties, including those discussed below and more fully described in the Securities and Exchange Commission reports filed by Amgen, including our most recent annual report on Form 10-K and any subsequent periodic reports on Form 10-Q and current reports on Form 8-K. Unless otherwise noted, Amgen is providing this information as of the date of this news release and does not undertake any obligation to update any forward-looking statements contained in this document as a result of new information, future events or otherwise.
No forward-looking statement can be guaranteed, and actual results may differ materially from those we project. Discovery or identification of new product candidates or development of new indications for existing products cannot be guaranteed and movement from concept to product is uncertain; consequently, there can be no guarantee that any particular product candidate or development of a new indication for an existing product will be successful and become a commercial product. Further, preclinical results do not guarantee safe and effective performance of product candidates in humans. The complexity of the human body cannot be perfectly, or sometimes, even adequately modeled by computer or cell culture systems or animal models. The length of time that it takes for us to complete clinical trials and obtain regulatory approval for product marketing has in the past varied and we expect similar variability in the future. Even when clinical trials are successful, regulatory authorities may question the sufficiency for approval of the trial endpoints we have selected. We develop product candidates internally and through licensing collaborations, partnerships and joint ventures. Product candidates that are derived from relationships may be subject to disputes between the parties or may prove to be not as effective or as safe as we may have believed at the time of entering into such relationship. Also, we or others could identify safety, side effects or manufacturing problems with our products, including our devices, after they are on the market.
Our results may be affected by our ability to successfully market both new and existing products domestically and internationally, clinical and regulatory developments involving current and future products, sales growth of recently launched products, competition from other products including biosimilars, difficulties or delays in manufacturing our products and global economic conditions. In addition, sales of our products are affected by pricing pressure, political and public scrutiny and reimbursement policies imposed by third-party payers, including governments, private insurance plans and managed care providers and may be affected by regulatory, clinical and guideline developments and domestic and international trends toward managed care and healthcare cost containment. Furthermore, our research, testing, pricing, marketing and other operations are subject to extensive regulation by domestic and foreign government regulatory authorities. Our business may be impacted by government investigations, litigation and product liability claims. In addition, our business may be impacted by the adoption of new tax legislation or exposure to additional tax liabilities. If we fail to meet the compliance obligations in the corporate integrity agreement between us and the U.S. government, we could become subject to significant sanctions. Further, while we routinely obtain patents for our products and technology, the protection offered by our patents and patent applications may be challenged, invalidated or circumvented by our competitors, or we may fail to prevail in present and future intellectual property litigation. We perform a substantial amount of our commercial manufacturing activities at a few key facilities, including in Puerto Rico, and also depend on third parties for a portion of our manufacturing activities, and limits on supply may constrain sales of certain of our current products and product candidate development. We rely on collaborations with third parties for the development of some of our product candidates and for the commercialization and sales of some of our commercial products. In addition, we compete with other companies with respect to many of our marketed products as well as for the discovery and development of new products. Further, some raw materials, medical devices and component parts for our products are supplied by sole third-party suppliers. Certain of our distributors, customers and payers have substantial purchasing leverage in their dealings with us. The discovery of significant problems with a product similar to one of our products that implicate an entire class of products could have a material adverse effect on sales of the affected products and on our business and results of operations. Our efforts to collaborate with or acquire other companies or products, and to integrate the operations of companies or in support of products we have acquired, may not be successful. A breakdown, cyberattack, or information security breach could compromise the confidentiality, integrity and availability of our systems and our data. Our stock price is volatile and may be affected by a number of events. Our business performance could affect or limit the ability of our Board of Directors to declare a dividend or our ability to pay a dividend or repurchase our common stock. We may not be able to access the capital and credit markets on terms that are favorable to us, or at all.
Any scientific information discussed in this news release relating to new indications for our products is preliminary and investigative and is not part of the labeling approved by the U.S. Food and Drug Administration for the products. The products are not approved for the investigational use(s) discussed in this news release, and no conclusions can or should be drawn regarding the safety or effectiveness of the products for these uses.