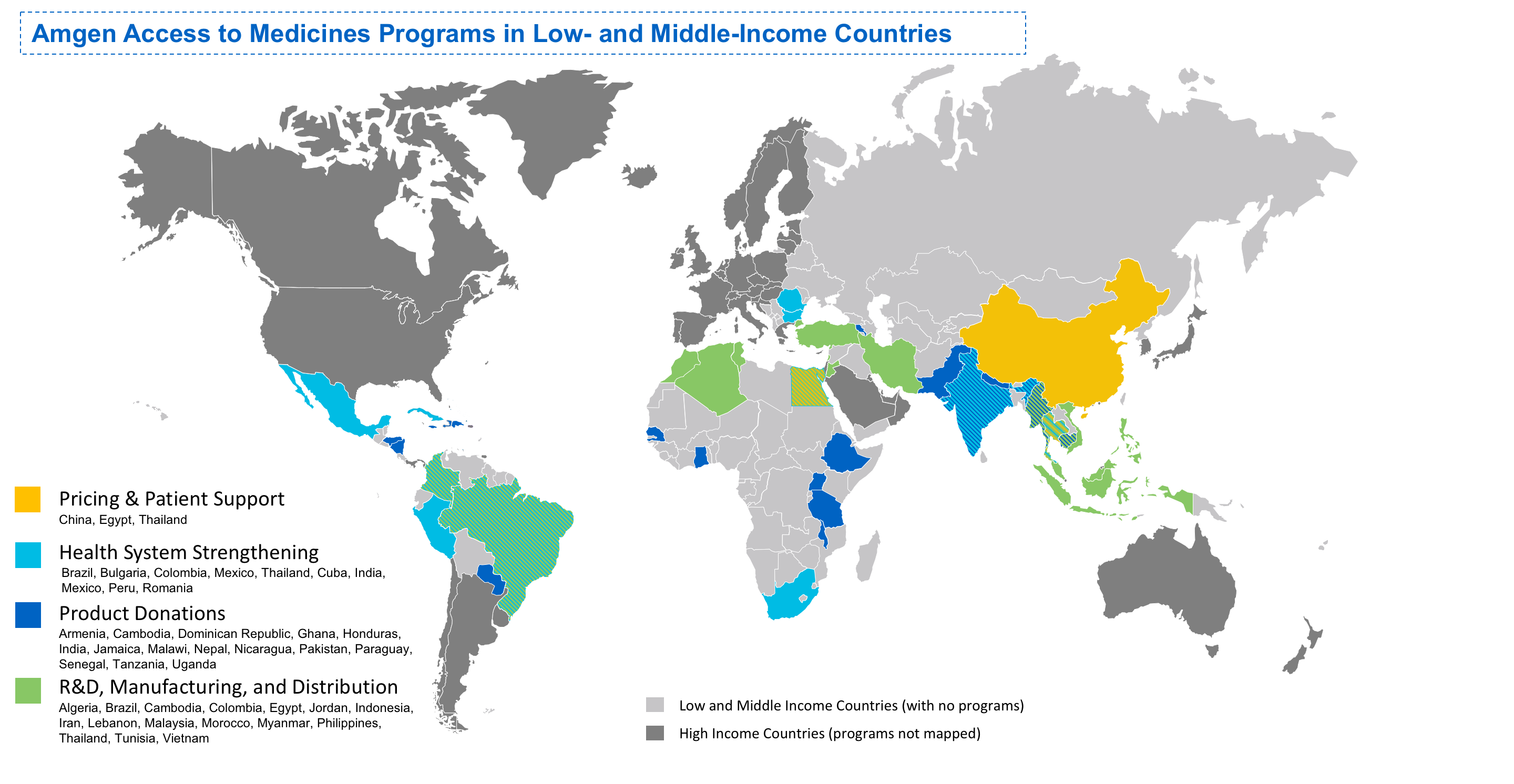
Access to Healthcare Initiatives Outside the U.S.
In light of the increasing global burden of cancer, cardiovascular disease, osteoporosis and other non-communicable diseases, Amgen's growing portfolio of biologic and biosimilar medicines holds the potential to make a significant difference for patients worldwide who are facing serious illnesses.
Amgen's access to healthcare approach is based on a recognition that patients living in low- and middle-income countries face multiple barriers to accessing biologic medicines. Those barriers include healthcare infrastructure and supply chain challenges to safely handle and administer biologics, as well as affordability in countries that lack adequate reimbursement and insurance systems.
Together with partners and stakeholders, we are working to overcome such access challenges and barriers through a multifaceted access to healthcare approach.
Our approach to access begins years before a product is approved. During all stages of drug development, a cross-functional team representing research, clinical development, commercial, medical affairs, and manufacturing works to define the potential new medicine's target profile – the attributes we want to build into the medicine – to help ensure we develop well-differentiated, accessible therapies that address unmet patient need for the countries in which we plan to launch. We also gather insights from external stakeholders to inform evidence needs for the global healthcare ecosystem.
Once approved, we use a variety of approaches to expand and evolve our efforts to assist more patients and increase access to our medicines globally.
Access to Medicines Structure and Management
Amgen's access to healthcare approach is a component of Amgen's ESG framework, which is governed by our cross-functional CEO-staff-level steering committee, the ESG Council. In addition, a cross-functional program review committee regularly reviews our access programs to ensure their compliance with all Amgen policies and local and national regulations.
The Corporate Responsibility and Compliance Committee of our Board of Directors receives regular updates from senior leaders about our approach to pricing, access and affordability, as well as on the activities of the Amgen Safety Net Foundation and other philanthropic activities that provide access to our medicines worldwide.
We implement our strategy through multiple functional groups including Global Value & Access; Corporate Affairs; Regulatory and Global Government Affairs & Policy; Advocacy, Value-Based Partnership team; our Biosimilars Division; Clinical Development, and our regional and local affiliate offices, which collaborate with local stakeholders to implement programs.