AMGen incorporates.
AMGen (Applied Molecular Genetics Inc.) is established in Thousand Oaks, California, on April 8, 1980, as the brainchild of venture capitalists William K. (Bill) Bowes and associates. With a staff of three, the Company occupies a shared building, now called “Building 1.”
Amgen has had three logos in its history. This is the first logo.
George B. Rathmann is named first CEO.
AMGen names its first CEO, scientist and businessman George B. Rathmann. Dubbed “Mr. Biotech” by Red Herring magazine, Rathmann has been called one of the great geniuses of high-tech entrepreneurialism. Working from a small trailer to free up space for scientists, Rathmann quickly establishes scientific goals and secures funding to conduct grand experiments in technology.
George Rathmann in one of the Building 2 laboratory bays in 1982.
Early experiments.
In the first three years, AMGen scientists attempt many things: creating organisms to extract oil from shale, growing chickens faster, making specialty chemicals, cloning luciferase (the light source for fireflies) and creating a process for producing indigo dye in E. coli—an achievement that garners the prestigious cover of Science magazine. The final direction for the Company would be treating and curing disease.
Amgen’s research in cloning genes leads to the Company's production of indigo in E. coli in the early 1980s. The discovery and subsequent patent made the cover of Science magazine in 1983.
Led by CFO Gordon Binder, Amgen’s IPO on June 17, 1983, raises nearly $40 million.
The Company officially changes its name to Amgen.
A May 1983 article of The Wall Street Journal announces Amgen’s IPO.
The clone that launched a company.
A team led by a young researcher from Taiwan named Fu-Kuen Lin is tasked with finding and cloning the erythropoietin gene. Their job is staggering: finding a gene on a single fragment of DNA among 1.5 million fragments of the human genome. After working tirelessly for two years, they do it. This groundbreaking achievement enables the creation of one of the most successful drugs in biotech history, EPOGEN® (epoetin alfa).
Fu-Kuen Lin examines x-ray film to identify gene coding for erythropoietin.
Kirin and Amgen form a joint venture, Kirin-Amgen, for the worldwide commercialization of erythropoietin.
A second discovery.
While Lin is working on erythropoietin, researcher Larry Souza and his team clone granulocyte colony-stimulating factor (G-CSF). This discovery leads to the development of Amgen’s second blockbuster drug NEUPOGEN® (filgrastim).
Research head Larry Souza leads the team in the creation of NEUPOGEN®, Amgen’s second blockbuster drug.
Gordon Binder is appointed CEO.
Amgen had just received the first U.S. patent for recombinant erythropoietin, completed a 20,000 page filing to the FDA for approval of EPOGEN® (epoetin alfa) and completed building a new 24,000 square foot manufacturing facility. At this height, Rathmann announces that he is ready to retire, explaining, “I figured that would be a great thing if they got a running start by launching EPOGEN® as the new management team.” Gordon Binder is promoted from CFO to CEO and ushers in a new and promising era.
Gordon Binder and Harry Hixson during the Company’s transition following George Rathmann’s retirement.
On June 1, 1989, the FDA approves EPOGEN® (epoetin alfa). EPOGEN® is named Product of the Year, by Fortune magazine.
Amgen goes international.
Amgen establishes its European headquarters in Lucerne, Switzerland. Later, the European headquarters will relocate to Zug, also in Switzerland. Over the next several years, Amgen would quickly establish offices across Europe, including a key manufacturing and distribution center in Breda, the Netherlands.
Aart Brouwer was the first head of Amgen’s European office from 1989 to 2001.
Giving back: the Amgen Foundation is founded.
Amgen establishes the Amgen Foundation as a way to to honor and amplify the existing culture of giving in the company, shaped by employees and groups who were already actively serving their communities.
Today, the Amgen Foundation works to advance science education, inspire the next generation of innovators, and support communities where Amgen employees live and work. By the end of 2025, the Foundation had donated more than $500 million in grants to local, regional and international nonprofit organizations that reflect Amgen’s core values and impact lives in inspiring and innovative ways.
Research director Frank Martin is just one of the many Amgen staffers who take their love of science to the classroom. In this 1995 photo, Martin guides students in an experiment at Walnut Elementary School in Thousand Oaks.
On February 21, 1991, NEUPOGEN® (filgrastim) is approved by the FDA.
NEUPOGEN® is named Product of the Year by Fortune magazine.
A billion-dollar company.
Amgen hits $1 billion in product sales for EPOGEN® (epoetin alfa) and NEUPOGEN® (filgrastim) combined. On January 2, 1992, Amgen is added to the S&P 500 and months later, the Company debuts on the Fortune 500 list.
Manufacturing staffers oversee a filling machine to dispense final, formulated product into vials ready for distribution.
Amgen opens its Puerto Rico facility, which would become Amgen’s flagship manufacturing site with over 1.7 million square feet of space.
Since 1993, Amgen has relied heavily on its facility in Puerto Rico.
Two important discoveries.
Amgen researcher Steve Elliott and his team add two sugar chains to erythropoietin, causing the protein to remain in the body longer. From this discovery, Aranesp® (darbepoetin alfa) is created. Around the same time, Amgen researcher Olaf Kinstler and his team are experimenting with a longer-lasting form of NEUPOGEN® (filgrastim). Amgen attaches the waxy, water-soluble polymer polyethylene glycol (PEG) to G-CSF, which expands the molecule and greatly slows down excretion. From this discovery, Neulasta® (pegfilgrastim) is created.
The top page of scientist Steve Elliott’s lab notebook contains early data from cells transformed with pDEC321, the plasmid used to construct the cell line that produces Aranesp®. With this data, he knew he’d found what he was seeking.
A time of scale and growth.
Staff numbers reach 3,396 globally, up from only 344 when Binder was named CEO in 1988. The Thousand Oaks headquarters had grown, too–from half a building in 1980 to a sprawling campus with well over a million square feet of space by 1992. Local newspapers describe the area as “perpetually under construction.”
In 1995, the Los Angeles Times writes a glowing article about Amgen’s relationship with the city of Thousand Oaks, calling it “a Match Made in Heaven.”
Amgen wins the National Medal of Technology.
Amgen becomes the first biotech company to receive the U.S. Department of Commerce National Medal of Technology. This award is considered by the U.S. government to be on par with the Nobel Prize. Given that year by Vice President Al Gore and Commerce Secretary Ron Brown, the award recognizes Amgen for “its leadership in developing innovative and important cost-effective therapeutics based on advances in the cellular and molecular biology for delivery to critically ill patients throughout the world.”
Vice President Al Gore presents the 1994 National Medal of Technology to CEO Gordon Binder. The award is the highest honor awarded by the President of the United States to America’s leading innovators.
The Amgen Values launch.
The Amgen Values are first launched. Amgen could not have accomplished what it has if not for its commitment to building a culture and social architecture that embraces science and innovation—a culture that continues to shape what Amgen is today.
Staffers help develop the principles that guide the way Amgen conducts business. This early mission statement has evolved into the current mission, aspiration, values and leadership attributes.
On November 2, 1998, the FDA approves Enbrel® (etanercept).
Key discoveries.
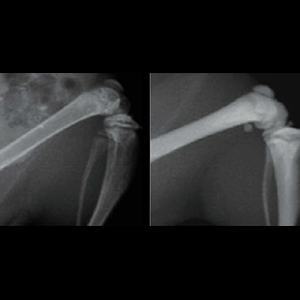
Scientists at Amgen identify and clone osteoprotegerin (OPG). Subsequent research showed that OPG functioned as a decoy receptor for RANK ligand. These insights form the scientific basis for denosumab.
An original x-ray from 1995.
A new CEO for a new century.
Kevin W. Sharer becomes Amgen’s third CEO, following the retirement of Gordon Binder. When Binder stepped down, Amgen had grown to become the fourteenth largest drug company in the world, having outstripped its early biotech rivals years before. As Amgen’s former President and COO, Sharer had split many responsibilities with Binder. Binder explained, “Like an athlete, there comes a time for the CEO to leave. We were about to launch preparations for several new products, and Kevin was ready to take command.”
Kevin Sharer and Amgen’s executive management team were featured in the July 2001 Pharmaceutical Executive magazine—the top trade journal in the industry.
Amgen’s Cambridge, Mass., research center opens.
Amgen becomes one of the early pioneers in what would become a biotechnology hotbed in Kendall Square, opening a 285,000-square-foot facility.
On September 17, 2001, Aranesp® (darbepoetin alfa) is approved by the FDA.
Amgen in space.
Amgen and NASA team up to study Amgen’s investigational treatment, osteoprotegerin (OPG), on the space shuttle Endeavour. The experiment mimics the effects of the rapid bone loss that astronauts experience due to microgravity.
NASA guest badges identify spectators at the liftoff.
Every patient, every time.
Amgen acquires Immunex, the developer of ENBREL® (etanercept), along with a manufacturing plant in Rhode Island that had not been used since being built 10 years earlier. Within a matter of months, Amgen teams secure FDA approval, start production and are able to manufacture enough ENBREL® to meet demand.
The Amgen Rhode Island manufacturing facility starts production with the creed:“We make ENBREL® so that no patient goes without.”
On January 31, 2002, Neulasta® (pegfilgrastim) is approved by the FDA.
On March 8, 2004, Sensipar® (cinacalcet) is approved by the FDA.
Amgen acquires Tularik, adding five candidates to Amgen’s pipeline and establishing a strong presence in South San Francisco.
Writing on a hood in a one of Amgen’s small molecule labs.
Elucidating the biology of PCSK9.
Scientists in Amgen’s labs in South San Francisco play a critical role in elucidating the function of PCSK9, which lays the groundwork for evolocumab.
A desktop in an Amgen lab in South San Francisco.
Breakaway from Cancer®.
Amgen founds Breakaway from Cancer, a national initiative to increase awareness of important resources available to people affected by cancer—from prevention through survivorship. Breakaway from Cancer represents a partnership between Amgen and four nonprofit organizations dedicated to empowering patients with education, resources and hope, wherever they may be in the cancer care continuum. Today, Breakaway from Cancer has reached hundreds of thousands of people touched by cancer with information about resources and services available to people affected by cancer, and more than $4 million has been donated to the Breakaway from Cancer nonprofit partners.
Actor and cancer patient advocate Patrick Dempsey participates in a Breakaway from Cancer® “Breakaway Mile” through Thousand Oaks honoring cancer survivors and their supporters during the 2011 Amgen Tour of California. Photo by Andy Tao.
On September 27, 2006, Vectibix® (panitumumab) is approved by the FDA
Women’s Genome Health Study begins.
Amgen collaborates with Brigham and Women’s Hospital and NIH’s National Heart, Lung, and Blood Institute on the Women’s Genome Health Study (WGHS). The purpose: to identify genetic variations that may underlie a range of serious illnesses including heart disease, stroke, diabetes, breast cancer and osteoporosis.
A strand of DNA. The initiative combs the DNA of 28,000 women, who donated their DNA to the groundbreaking study, for differences between those who have developed serious illness and those who have remained healthy.
Providing cutting-edge research experiences.
The Amgen Foundation, in collaboration with Massachusetts Institute of Technology (MIT), launches the Amgen Scholars program to provide undergraduates with access to research experiences and exposure to biotechnology and drug discovery at top institutions globally.
As an Amgen Scholar in 2013, Maithreyi Raman had the opportunity to conduct research on Huntington’s disease in Professor Franz-Ulrich Hartl’s laboratory at Ludwig-Maximilians-Universität in Germany.
On August 22, 2008, Nplate® (romiplostim) is approved by the FDA.
Nplate® (romiplostim) is awarded “Best Biotechnology Product” by Prix Galien.
Prix Galien is an international award that recognizes outstanding achievements in improving the human condition through the development of innovative therapies.
Prolia® (denosumab) and XGEVA® (denosumab) are approved by the FDA on June 1, 2010 and November 18, 2010, respectively.
Prolia® wins Best New Drug from Scrip, one of the industry’s highest global accolades.
Prolia® (denosumab) and XGEVA® (denosumab) are awarded “Best Biotechnology Product” by Prix Galien.
Prix Galien is an international award that recognizes outstanding achievements in improving the human condition through the development of innovative therapies.
Amgen, CDC and CDC Foundation partner to improve infection control for cancer patients.
Preventing Infections in Cancer Patients is a comprehensive public health collaboration between the CDC, the CDC Foundation and Amgen to help reduce infections by raising awareness among patients, caregivers and healthcare providers about steps they can take to protect themselves during chemotherapy treatment. Program resources include a Basic Infection Control and Prevention Plan for outpatient oncology clinics and an online patient risk assessment tool, in addition to posters, fact sheets and postcards. As of 2015, nearly 650,000 pieces of initiative materials have been disseminated to the public.
Tapping Amgen’s biomanufacturing expertise to create biosimilars.
Amgen and Actavis, Inc. announce that they will collaborate to develop and commercialize, on a worldwide basis, several oncology antibody biosimilar medicines. This collaboration reflects the shared belief that the development and commercialization of biosimilar products will not follow a pure brand or generic model, and will require significant expertise, infrastructure and investment to ensure safe, reliably supplied therapies for patients.
Manufacturing equipment in Rhode Island.
Amgen acquires a manufacturing facility near Dublin, Ireland.
Amgen acquires BioVex, developers of talimogene laherparepvec.
A T cell.
Amgen expands in Brazil, including the acquisition of Bergamo, a privately held Brazilian pharmaceutical company.
Robert A. Bradway is appointed as Amgen’s fourth CEO.
After more than a decade of leading Amgen as the world’s largest biotechnology company, Sharer announces his retirement and that the reins will be handed over to Bradway, Amgen's president and COO. Vance Coffman, chairman of the board's governance and nominating committee at that time, explains, "During [Kevin’s tenure], Amgen grew significantly in every dimension and is well positioned for the future."
Robert A. Bradway
Strategic partners and acquisitions.
Amgen acquires deCODE Genetics, a global leader in human genetics. The acquisition reflects a core tenet of Amgen’s current R&D strategy: finding and pursuing drug targets that are validated by human genetics. That same year, Amgen and AstraZeneca agree to jointly develop and commercialize five monoclonal antibodies from Amgen's inflammation portfolio. Amgen also acquires Micromet Inc., developers of what would later be approved by the FDA as BLINCYTO® (blinatumomab); KAI Pharmaceuticals, developers of AMG 416; and Mustafa Nevzat, a leading privately held Turkish pharmaceutical company.
Amgen Teach launches in Europe.
Amgen Teach launches in Europe to provide hundreds of science educators with free training sessions that emphasize hands-on, inquiry-based experiential learning for their students.
Science teacher Kirstie McAdoo of Ireland shares that participating in Amgen Teach "has given me a huge amount of confidence to use inquiry-based learning in the classroom."
Amgen Astellas BioPharma K.K. alliance forms in Japan and Amgen-Betta Pharmaceuticals joint venture is established in China.
Amgen acquires Onyx Pharmaceuticals, developers of Kyprolis® (carfilzomib) for Injection.
Through a collaboration with Servier, Amgen obtains the U.S. commercial rights to ivabradine.
On December 3, 2014, BLINCYTO® (blinatumomab) is approved by the FDA.
The next generation of biomanufacturing.
Construction is completed on a state-of-the-art facility in Singapore. The plant has the same capacity as a conventional plant, but in a smaller space, using less water and less energy while producing fewer solid wastes and fewer emissions.
The groundbreaking ceremony on June 3, 2013, for the Singapore manufacturing facility.
Amgen’s Asia Research and Development Center opens at ShanghaiTech University in China.
The R&D Center’s opening ceremony.
On March 2, 2015, the Neulasta® (pegfilgrastim) Delivery Kit, including the On-body Injector, launches.
On April 15, 2015, Corlanor® (ivabradine) is approved by the FDA.
On August 27, 2015, Repatha® (evolocumab) is approved by the FDA.
On October 27, 2015, IMLYGIC™ (talimogene laherparepvec) is approved by the FDA.
Amgen Singapore Manufacturing Facility
Amgen's first commercial chemical synthesis manufacturing facility opens in Singapore. The facility is in line with Amgen’s global expansion strategy to strengthen its biomanufacturing capabilities.
On July 11, 2016, the Repatha® (evolocumab) Pusthronex™ system (on-body infusor with prefilled cartridge) is approved.
On September 23, 2016, AMJEVITA™ (Adalimumab-Atto) is approved by the FDA. It is Amgen's first biosimilar to receive regulatory approval.
On February 7, 2017, Parsabiv® (etelcalcetide) is approved by the FDA.
On September 14, 2017, MVASI™ (bevacizumab-awwb) is approved by the FDA.
ACC Opens in Tampa, FL
In October 2017, Amgen Capability Center (ACC) opens in Tampa, Florida.
On November 11, 2017, Amgen launches the ENBREL (etanercept) Mini™ single-dose prefilled cartridge with AutoTouch™ Reusable Autoinjector that is ergonomically designed for patients.
On May 17, 2018, Aimovig™ (erenumaab-aooe) is approved by the FDA.
Amgen Rhode Island Breaks Ground on First U.S. Next-Gen Plant
Amgen breaks ground on its new next-generation biomanufacturing plant at its West Greenwich, Rhode Island campus. The new plant will be the first of its kind in the U.S., designed for greater flexibility, speed and efficiency, and will manufacture products for the U.S. and global markets.
On April 9, 2019, EVENITY™ (romosozumab-aqqg) is approved by the FDA.
On June 13, 2019, KANJINTI™ (trastuzumab-anns) is approved by the FDA.
On November 21, 2019, Amgen completes the acquisition of Otezla® (apremilast).
On December 6, 2019, AVSOLA™ (infliximab-axxq) is approved by the FDA.
Amgen Closes a Strategic Collaboration with BeiGene
On January 2, 2020, Amgen announces a strategic collaboration with BeiGene to expand its oncology presence in China.

Amgen Foundation and Harvard Launch "LabXchange"
Amgen Foundation and Harvard announce the global launch of LabXchange™, a free online science education platform.

Amgen Establishes Wholly-Owned Affiliate In Japan
Amgen Astellas BioPharma K.K. in Japan becomes wholly owned affiliate, and renamed Amgen K.K.

Amgen Turns 40
Over 40 years, Amgen grew from a small team in a California office park to a global biotech leader in approximately 100 countries. Hundreds of biotech companies have come and gone since our founding in 1980, but Amgen is still here, and we're stronger than ever.

Amgen Joins the Dow Jones Industrial Average
On August 24, 2020, Amgen joins the Dow Jones Industrial Average.

FDA Approval of RIABNI™ as biosimilar to Rituxan®
On Decmber 17, 2020, the FDA approved Amgen's RIABNI™ (rituximab-arrx) as a biosimilar to Rituxan® (rituximab).

Acquisition of Five Prime Therapeutics
On April 16, 2021, Amgen successfully completed its acquisition of Five Prime Therapeutics.

FDA Approval of LUMAKRAS™ for KRAS G12C-mutated Non-Small Cell Lung Cancer (NSCLC)
On May 28, 2021, the FDA approved Amgen's LUMAKRAS™ (sotorasib) as the first and only targeted treatment for patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer (NSCLC).

Acquisition of Teneobio, Inc
On October 19, 2021, Amgen successfully completed its acquisition of Teneobio, Inc.

Tezspire™ Approved by FDA for Severe Asthma
On December 17, 2021, the FDA approved Amgen and AstraZeneca's Tezspire™ (tezepelumab-ekko) in the U.S. for severe asthma.

Acquisition of Chemocentryx
On October 20, 2022, Amgen successfully completed its acquisition of Chemocentryx.

AMJEVITA™ Becomes Available in U.S.
On January 31, 2023, Amgen's AMJEVITA™ (adalimumab-atto), the first biosimilar to Humira® (adalimumab), became available in the U.S. It had been approved by the FDA in 2016.

Amgen Expands into Rare Disease with Acquisition of Horizon Therapeutics plc
On October 6, 2023, Amgen completed its acquisition of Horizon Therapeutics plc, marking the creation of Amgen's Rare Disease business.

Amgen Ohio Opens
On February 26, 2024, Amgen opened a new, state-of-the-art biomanufacturing site in Central Ohio.

IMDELLTRA™ (tarlatamab-dlle) Approved by FDA for ES-SCLC
On May 16, 2024, the FDA approved Amgen's IMDELLTRA™ (tarlatamab-dlle) for the treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).