It’s been 30 years since Peter Kufer, M.D., vice president of Bispecific T-cell Engager (BiTE®) technology at Amgen, first presented BiTE science to the research community. Over the last three decades, scientists around the world have worked diligently to advance this ground-breaking technology, and their unwavering dedication persists in striving to help those impacted by difficult-to-treat cancers.
BLINCYTO® (blinatumomab), the first globally approved BiTE immuno-oncology therapy, recently received U.S. Food and Drug Administration (FDA) approval for its third indication in the treatment of adult and pediatric patients one month or older with CD19-positive Philadelphia-chromosome-negative B-cell precursor acute lymphoblastic leukemia (B-ALL) in the consolidation phase, regardless of measurable residual disease (MRD) status.
“BiTE therapy has catalyzed a significant shift in how we approach the treatment of B-ALL, an aggressive form of blood cancer that commonly relapses after initial treatment,” explains Jay Bradner, M.D., executive vice president, Research and Development, and chief scientific officer at Amgen.1,2 “With this latest approval, the use of BLINCYTO at the outset of B-ALL therapy in the consolidation phase offers hope of achieving a long lasting remission.”
INDICATIONS
BLINCYTO® (blinatumomab) is indicated for the treatment of CD19-positive B-cell precursor acute lymphoblastic leukemia (ALL) in adult and pediatric patients one month and older with:
- Philadelphia chromosome-negative disease in the consolidation phase of multiphase chemotherapy
- Minimal residual disease (MRD) greater than or equal to 0.1% in first or second complete remission
- Relapsed or refractory disease
IMPORTANT SAFETY INFORMATION
WARNING: CYTOKINE RELEASE SYNDROME and NEUROLOGICAL TOXICITIES including IMMUNE EFFECTOR CELL-ASSOCIATED NEUROTOXICITY SYNDROME
- Cytokine Release Syndrome (CRS), which may be life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® and treat with corticosteroids as recommended.
- Neurological toxicities, including immune effector cell-associated neurotoxicity syndrome (ICANS) which may be severe, life-threatening or fatal, occurred in patients receiving BLINCYTO®. Interrupt or discontinue BLINCYTO® as recommended.
Please see additional Important Safety Information below.
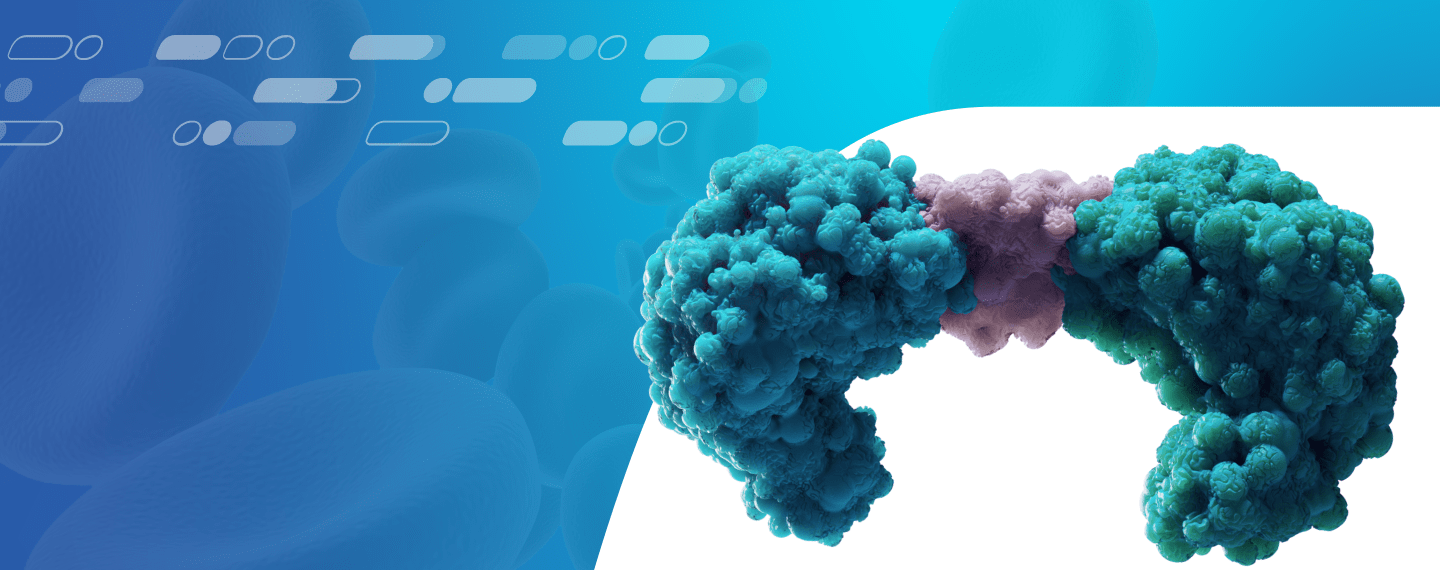
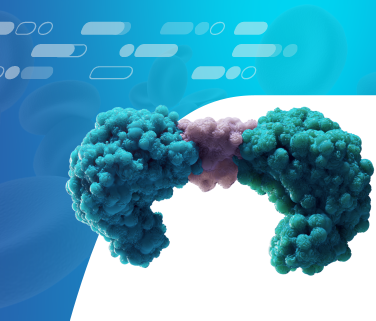
How BiTE Technology Strategically Targets Cancer
Amgen’s BiTE technology is designed to engage the body’s immune system by using patients’ own T cells to target cancer cells directly.3 Cytotoxic T cells identify and help fight cancer cells. However, cancer cells have the ability to develop mechanisms that allow them to evade T cell recognition and destruction.3,4 The BiTE molecule is engineered to have an arm that binds with a protein found on the surface of the T cell and another arm that binds to a specific protein found primarily on cancerous tumor cells.5

Maximizing Therapeutic Potential
BiTE therapies have already had a profound impact on patients worldwide, but there is potential to reach many more patients – including those with rare and aggressive cancers who have historically poor outcomes.
“Our strides with BiTE therapy and BLINCYTO have been transformative, yet our journey persists,” said Kufer. “Leveraging our extensive data empowers us to further advance the development of BiTE medicines, prioritize the patient experience and continue to explore novel approaches for diverse hematologic and solid tumor types.”
To learn more about Amgen's latest approval for BLINCYTO, click here.
BLINCYTO® IMPORTANT SAFETY INFORMATION (continued)
Contraindications
BLINCYTO® is contraindicated in patients with a known hypersensitivity to blinatumomab or to any component of the product formulation.
Warnings and Precautions
-
Cytokine Release Syndrome (CRS): CRS, which may be life-threatening or fatal, occurred in patients receiving BLINCYTO®. The median time to onset of CRS is 2 days after the start of infusion and the median time to resolution of CRS was 5 days among cases that resolved. Closely monitor and advise patients to contact their healthcare professional for signs and symptoms of serious adverse events such as fever, headache, nausea, asthenia, hypotension, increased alanine aminotransferase (ALT), increased aspartate aminotransferase (AST), increased total bilirubin, and disseminated intravascular coagulation (DIC). The manifestations of CRS after treatment with BLINCYTO® overlap with those of infusion reactions, capillary leak syndrome (CLS), and hemophagocytic histiocytosis/macrophage activation syndrome (MAS). Using all of these terms to define CRS in clinical trials of BLINCYTO®, CRS was reported in 15% of patients with R/R ALL, in 7% of patients with MRD-positive ALL, and in 16% of patients receiving BLINCYTO® cycles in the consolidation phase of therapy. If severe CRS occurs, interrupt BLINCYTO® until CRS resolves. Discontinue BLINCYTO® permanently if life-threatening CRS occurs. Administer corticosteroids for severe or life-threatening CRS.
-
Neurological Toxicities, including Immune Effector Cell-Associated Neurotoxicity Syndrome: BLINCYTO® can cause serious or life-threatening neurologic toxicity, including ICANS. The incidence of neurologic toxicities in clinical trials was approximately 65%. The median time to the first event was within the first 2 weeks of BLINCYTO® treatment. The most common (≥ 10%) manifestations of neurological toxicity were headache and tremor. Grade 3 or higher neurological toxicities occurred in approximately 13% of patients, including encephalopathy, convulsions, speech disorders, disturbances in consciousness, confusion and disorientation, and coordination and balance disorders. Manifestations of neurological toxicity included cranial nerve disorders. The majority of neurologic toxicities resolved following interruption of BLINCYTO®, but some resulted in treatment discontinuation.
The incidence of signs and symptoms consistent with ICANS in clinical trials was 7.5%. The onset of ICANS can be concurrent with CRS, following resolution of CRS, or in the absence of CRS. There is limited experience with BLINCYTO® in patients with active ALL in the central nervous system (CNS) or a history of neurologic events. Patients with a history or presence of clinically relevant CNS pathology were excluded from clinical studies. Patients with Down Syndrome over the age of 10 years may have a higher risk of seizures with BLINCYTO® therapy.
Monitor patients for signs and symptoms of neurological toxicities, including ICANS, and interrupt or discontinue BLINCYTO® as outlined in the PI. Advise outpatients to contact their healthcare professional if they develop signs or symptoms of neurological toxicities.
-
Infections: Approximately 25% of patients receiving BLINCYTO® in clinical trials experienced serious infections such as sepsis, pneumonia, bacteremia, opportunistic infections, and catheter-site infections, some of which were life-threatening or fatal. Administer prophylactic antibiotics and employ surveillance testing as appropriate during treatment. Monitor patients for signs or symptoms of infection and treat appropriately, including interruption or discontinuation of BLINCYTO® as needed.
-
Tumor Lysis Syndrome (TLS), which may be life-threatening or fatal, has been observed. Preventive measures, including pretreatment nontoxic cytoreduction and on-treatment hydration, should be used during BLINCYTO® treatment. Monitor patients for signs and symptoms of TLS and interrupt or discontinue BLINCYTO® as needed to manage these events.
-
Neutropenia and Febrile Neutropenia, including life-threatening cases, have been observed. Monitor appropriate laboratory parameters (including, but not limited to, white blood cell count and absolute neutrophil count) during BLINCYTO® infusion and interrupt BLINCYTO® if prolonged neutropenia occurs.
-
Effects on Ability to Drive and Use Machines: Due to the possibility of neurological events, including seizures and ICANS, patients receiving BLINCYTO® are at risk for loss of consciousness, and should be advised against driving and engaging in hazardous occupations or activities such as operating heavy or potentially dangerous machinery while BLINCYTO® is being administered.
-
Elevated Liver Enzymes: Transient elevations in liver enzymes have been associated with BLINCYTO® treatment with a median time to onset of 3 days. In patients receiving BLINCYTO®, although the majority of these events were observed in the setting of CRS, some cases of elevated liver enzymes were observed outside the setting of CRS, with a median time to onset of 19 days. Grade 3 or greater elevations in liver enzymes occurred in approximately 7% of patients outside the setting of CRS and resulted in treatment discontinuation in less than 1% of patients. Monitor ALT, AST, gamma-glutamyl transferase, and total blood bilirubin prior to the start of and during BLINCYTO® treatment. BLINCYTO® treatment should be interrupted if transaminases rise to > 5 times the upper limit of normal (ULN) or if total bilirubin rises to > 3 times ULN.
-
Pancreatitis: Fatal pancreatitis has been reported in patients receiving BLINCYTO® in combination with dexamethasone in clinical trials and the post-marketing setting. Evaluate patients who develop signs and symptoms of pancreatitis and interrupt or discontinue BLINCYTO® and dexamethasone as needed.
-
Leukoencephalopathy: Although the clinical significance is unknown, cranial magnetic resonance imaging (MRI) changes showing leukoencephalopathy have been observed in patients receiving BLINCYTO®, especially in patients previously treated with cranial irradiation and antileukemic chemotherapy.
-
Preparation and administration errors have occurred with BLINCYTO® treatment. Follow instructions for preparation (including admixing) and administration in the PI strictly to minimize medication errors (including underdose and overdose).
-
Immunization: Vaccination with live virus vaccines is not recommended for at least 2 weeks prior to the start of BLINCYTO® treatment, during treatment, and until immune recovery following last cycle of BLINCYTO®.
-
Benzyl Alcohol Toxicity in Neonates: Serious adverse reactions, including fatal reactions and the “gasping syndrome”, have been reported in very low birth weight (VLBW) neonates born weighing less than 1500 g, and early preterm neonates (infants born less than 34 weeks gestational age) who received intravenous drugs containing benzyl alcohol as a preservative. Early preterm VLBW neonates may be more likely to develop these reactions, because they may be less able to metabolize benzyl alcohol.
Use the preservative-free preparations of BLINCYTO® where possible in neonates. When prescribing BLINCYTO® (with preservative) for neonatal patients, consider the combined daily metabolic load of benzyl alcohol from all sources including BLINCYTO® (with preservative), other products containing benzyl alcohol or other excipients (e.g., ethanol, propylene glycol) which compete with benzyl alcohol for the same metabolic pathway.
Monitor neonatal patients receiving BLINCYTO® (with preservative) for new or worsening metabolic acidosis. The minimum amount of benzyl alcohol at which serious adverse reactions may occur in neonates is not known. The BLINCYTO® 7-Day bag (with preservative) contains 7.4 mg of benzyl alcohol per mL.
-
Embryo-Fetal Toxicity: Based on its mechanism of action, BLINCYTO® may cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to the fetus. Advise females of reproductive potential to use effective contraception during treatment with BLINCYTO® and for 48 hours after the last dose.
Adverse Reactions
The safety of BLINCYTO® in adult and pediatric patients one month and older with MRD-positive B-cell precursor ALL (n=137), relapsed or refractory B-cell precursor ALL (n=267), and Philadelphia chromosome-negative B cell precursor ALL in consolidation (n=165) was evaluated in clinical studies. The most common adverse reactions (≥ 20%) to BLINCYTO® in this pooled population were pyrexia, infusion-related reactions, headache, infection, musculoskeletal pain, neutropenia, nausea, anemia, thrombocytopenia, and diarrhea.
Dosage and Administration Guidelines
BLINCYTO® is administered as a continuous intravenous infusion at a constant flow rate using an infusion pump which should be programmable, lockable, non-elastomeric, and have an alarm.
It is very important that the instructions for preparation (including admixing) and administration provided in the full Prescribing Information are strictly followed to minimize medication errors (including underdose and overdose).
Please see BLINCYTO® full Prescribing Information, including BOXED WARNINGS.
USA-103-81591
References:
- National Cancer Institute. Precursor B-lymphoblastic leukemia. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/precursor-b-lymphoblastic-leukemia. Accessed February 26, 2024.
- Tian Z, et al. J Hematol Oncol. 2021;14:75.
- Baeuerle PA, et al. Curr Opin Mol Ther. 2009;11:22-30.
- Ferrone S, et al. Surg Oncol Clin N Am. 2007;16:755-774.
- Einsele H, et al. Cancer. 2020;126(14):3192-3201.