Despite the ever-increasing sophistication of modern medicine, it is often surprisingly hard for doctors to answer the most common questions that patients ask.
“Patients want to know what is going to happen to them, whether their disease is going to go away, or worsen, and they want to know whether or not they will benefit from a therapy,” said Rob Lenz, senior VP, R&D Global Development at Amgen. “I remember countless conversations I had as a physician. My patients wanted certainty. All I could give them was uncertainty.”
Scientists at Amgen believe greater certainty can be found by diving deeper into the oceans of human data contained within each of us. There’s enough raw information in a single human genome to fill a small library—and genomics, the study of our DNA, is just one branch in the burgeoning field of science informally known as “omics.” The genes in our DNA are like unspoken words until they’re transcribed into RNA, and the science of transcriptomics can tell us which genes are being expressed and which are silent. RNA, in turn, provides the template cells use to assemble the myriad proteins that drive biology, and proteomics now offers a detailed census of proteins present in blood or tissue.
The data points flowing from various branches of omics are like pixels that can be pieced together to build a sharper picture of how disease takes root and develops. The technology has the potential to deliver new biomarkers for earlier diagnosis of disease, faster and more successful clinical trials, and the ability to predict and prevent disease before it becomes more grievous and costly.
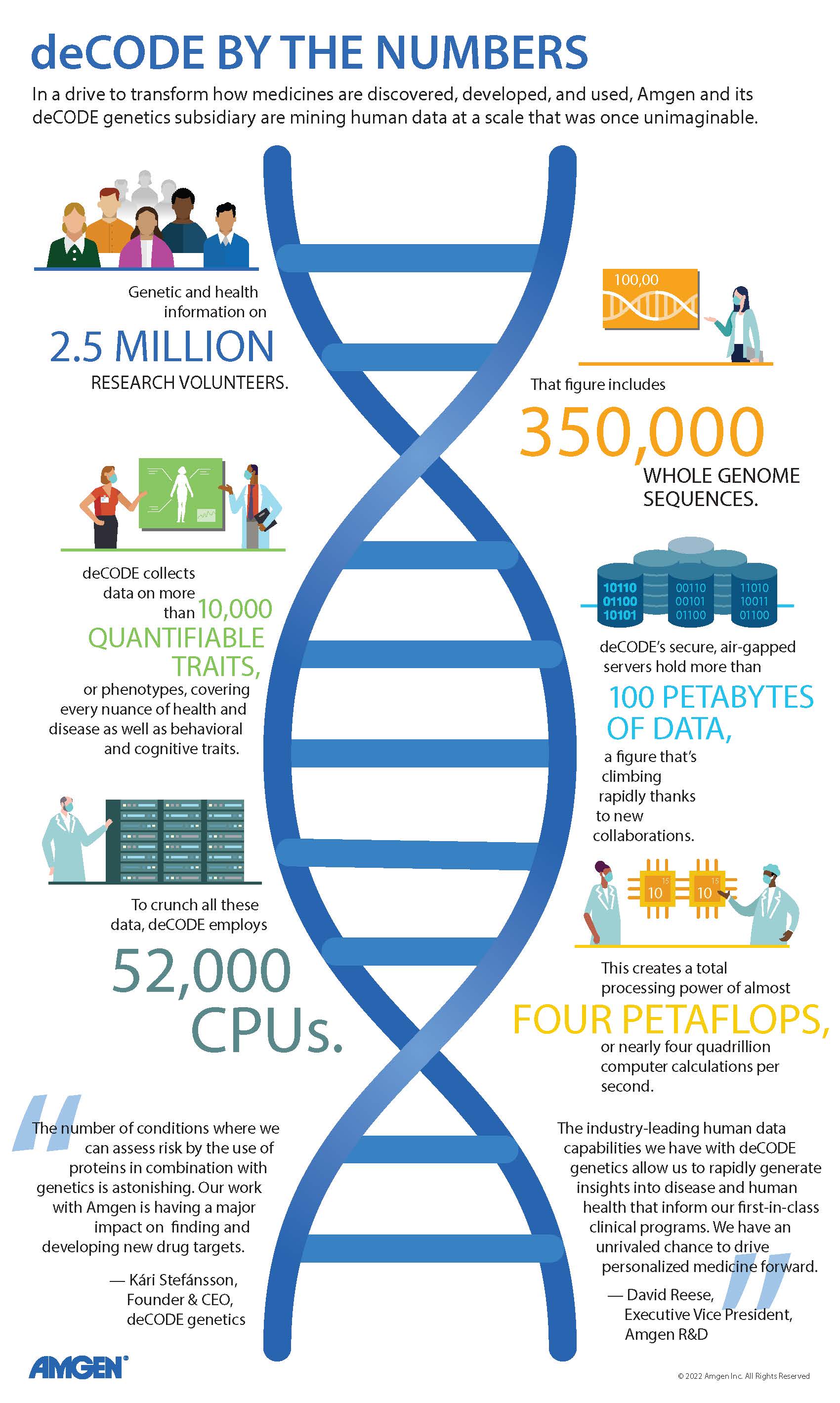
deCODE’s rapidly growing data pipeline
In the quest to apply a multi-omics approach to drug discovery and development, Amgen has had a head start thanks to its deCODE genetics subsidiary in Iceland. Genomics is the most mature branch of omics, and under founder Kári Stefánsson, deCODE had already built a world-class reputation for gene discovery before its 2012 acquisition by Amgen. The company pioneered the concept of collecting large-scale genetic and health data and using sophisticated statistical methods to find significant links between these two data sets.

deCODE's genome sequencing lab
“It is easy to forget that when Kári founded deCODE Genetics, these concepts were considered quite radical and unlikely to succeed. He was both literally and figuratively on a small island of his own,” said Mark Daly, a director of the Institute for Molecular Medicine Finland and co-director of the Broad Institute’s Program in Medical and Population Genetics. “Kári and his impressive team have made a massively outsized contribution to human genetics.”
In the years since the Amgen acquisition, deCODE has expanded its groundbreaking research well beyond its Icelandic base. “The principal task of human genetics is the study of human diversity, and the diversity in Iceland is somewhat limited because we are a small and relatively homogenous population,” said Kári, “To capture more rare variants in the sequence of the genome that affect risk of disease, we have extended our work outside of Iceland and now have over two and a half million people from all over the world participating in our research.”
That includes the 500,000 participants in the UK Biobank, a research charity that is following the health of its many older volunteers to improve disease diagnosis, treatment, and prevention. The Whole Genome Sequencing Project—the largest effort of its kind yet undertaken—aims to read the full genomes of all half million UK Biobank participants. In September 2019, deCODE was selected as one of just two sequencing providers for the project, along with the Wellcome Sanger Institute in the UK. Amgen is one of four corporate partners helping to fund the project, which also has the support of the UK government and the Wellcome Trust. Earlier in 2019, deCODE and Intermountain Healthcare, a Utah-based healthcare delivery network, announced a separate collaboration to analyze the genomes of up to 500,000 Intermountain patients.
“The reason we can do this work today,” observed Kári, “is that we can gather, we can store, and we can mine data of a magnitude that was inconceivable only ten or 15 years ago.” (See “deCODE by the Numbers”)
As deCODE’s data pipeline has expanded, so has its role within Amgen’s R&D strategy. “When we acquired deCODE in 2012, we were almost exclusively focused on how they could help us to discover and evaluate drug targets through human genetics,” said David Reese, Amgen’s executive vice president for R&D. “Now, we’re layering in genetics and other types of human data across the whole spectrum of R&D challenges, from guiding our insights into disease biology through drug safety and how we design clinical trials.
“We believe we have the right sequencing collaborations in place now to provide the breadth and diversity of genetic data we need to make the discoveries we are pursuing,” Reese added. “Now, we want to go from scale to depth by characterizing the biology of research participants in greater detail.”
From genes to proteins
The technology required to add that depth has made rapid progress in recent years. Proteomics, for example, can now identify proteins found at miniscule levels in the blood.
“The proteins we really want to measure—the cytokines with profound impacts on biological systems—these are present at much lower levels than the proteins that get measured in a standard blood test,” said Ray Deshaies, senior VP of Global Research at Amgen. “In fact, the total variation from the least abundant proteins in blood to the most abundant protein, albumin, is 10 billion-fold. Fortunately, there are now companies that can measure proteins across that whole range.”
In what may be the largest proteomics experiment to date, deCODE and a collaborator measured the relative levels of roughly 5,000 different proteins in plasma collected from 37,000 Icelanders whose genomes had already been sequenced. The early results from this project and similar research now underway at Amgen “are yielding all kinds of exciting observations,” said Kári.
“On the basis of proteins in plasma, we can identify individuals who are at high probability of dying within the next five years,” he added. “By combining genetics and proteins, we can find people with extraordinary risk of heart attack. The number of conditions where you can assess risk by the use of proteins in combination with genetics is astonishing. This is going to have a major impact on our ability to find new drug targets. It will significantly change the way in which clinical trials are designed and interpreted.”
Smaller clinical trials with bigger results
That change has already begun at Amgen, where drug development teams are starting to use genetic risk scores to identify the patients most likely to need and benefit from new investigational therapies. These polygenic scores measure the risk of developing a disease based on the total number of genetic changes related to that disease.
“We can use polygenic risk scores to ensure we’re not mixing patients with very different types or degrees of risk in the same study,” said Lenz. “We can also use them to identify and enroll high-risk patients who might benefit most from a test drug if it’s effective. A drug’s treatment effect should emerge sooner and more clearly in patients at greater risk for poor outcomes.”
Genetic risk scores, augmented by proteomics, could reduce the uncertainties that inflate the size, duration, and cost of trials, especially cardiovascular outcomes studies. “In the first year after a heart attack, only about 10 percent of patients will have another event,” Lenz observed. “Until now, companies haven’t had a good way to identify these very high-risk patients, so we enroll people who have had a previous heart attack in our studies, knowing we can only prevent a subsequent heart attack in the small fraction of these patients who will actually have one.
“With genetics and related data, we will get much better at distinguishing different levels of risk, and that will make a huge difference. It will allow us to dramatically reduce the size and duration of our trials, increase the probability of success and the size of the treatment effect, and get our medicines approved and into the hands of the patients who need them much sooner.”
Pivoting in response to COVID-19
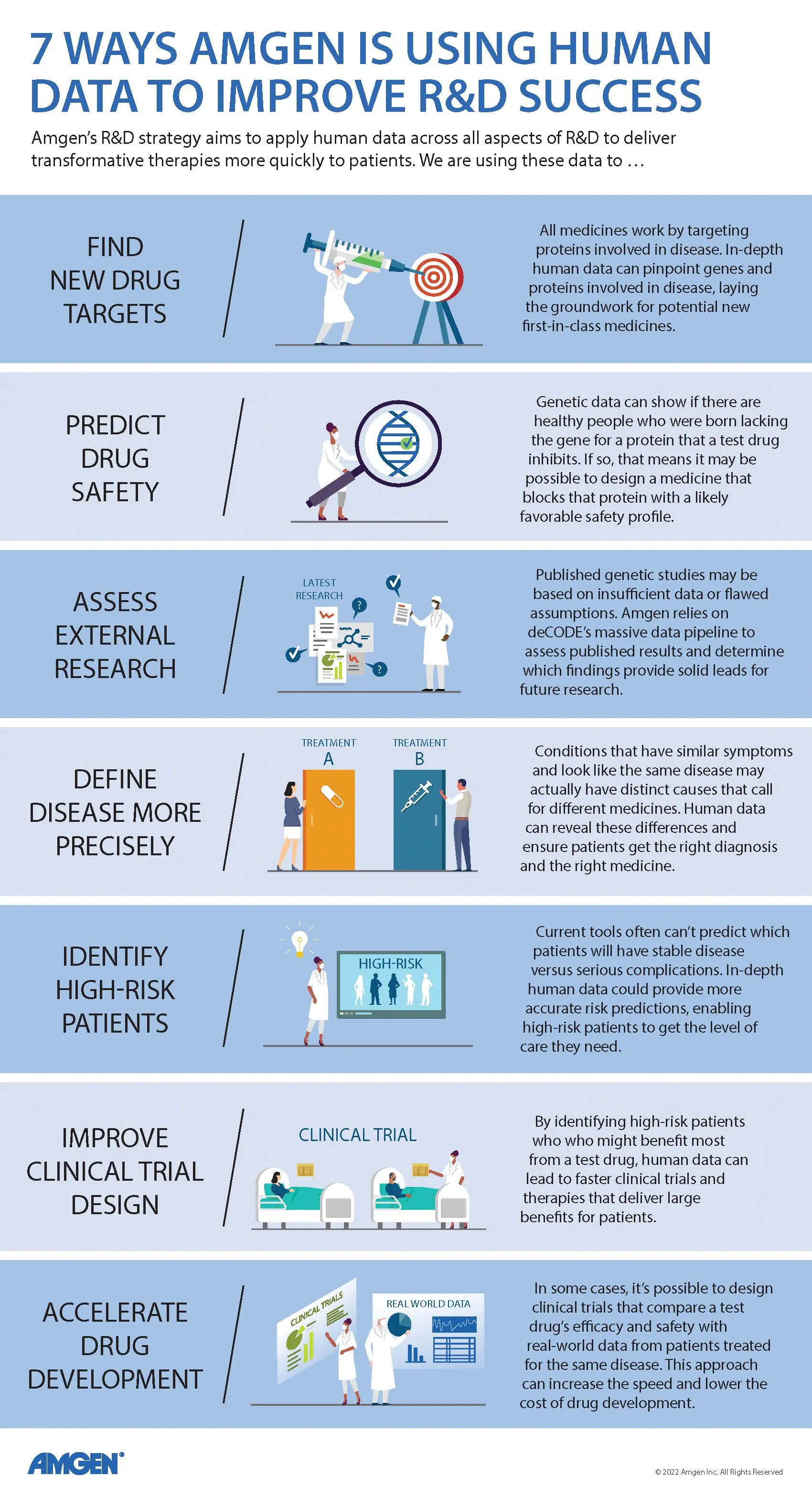
Improved trial design is just one of multiple ways that human data is already being applied to support R&D at Amgen. Scientists there have relied on their deCODE counterparts for insights into the quality of published genetic research and the potential safety implications of drug targets (see “7 Ways Amgen Is Using Human Data to Improve R&D Success”).
In addition to supporting Amgen’s work, deCODE scientists have maintained an interest in basic genetic research, publishing groundbreaking research on topics as diverse as the role of Neanderthal DNA in modern genomes and new ways to assess how genetic and environmental factors contribute to complex traits. In 2020, the year of COVID-19, deCODE pivoted to address the pandemic in Iceland, tracking the spread of coronavirus by studying genetic mutations in SARS-CoV-2 and measuring the durability of human antibodies to the virus. deCODE’s role in Iceland’s pandemic response wasn’t limited to research. With Amgen’s full support, the company used its DNA sequencing capabilities to help contain the virus in Iceland by testing tens of thousands of people.
“This team has been working together for almost a quarter of a century, and it is almost like everything else we have done, it feels like it was merely a preparation for this,” said Kári. “We have enormous amounts of data on the people who got infected. We are in a privileged position to look at the genetics of the patients and to see how the genetics influences the probability of getting infected and the probability of getting seriously ill when you become infected.”
“The ultimate in personalized medicine”
Human data isn’t a cure-all for the risks inherent in biopharma research. While it is estimated that genetic support can boost a drug’s odds of success in the clinic from 10 percent to 20 percent, that improvement is hardly a guarantee against failure. This point was underscored by the lack of success at Amgen and elsewhere in investigating the prevention of Alzheimer’s disease by inhibiting BACE, a target with robust genetic validation. While Alzheimer’s has been an especially difficult challenge, scientists face the same basic hurdle in any disease they study.
“I came to Amgen from academia, and my early training was as a yeast geneticist,” said Deshaies. “One thing I learned from that experience is that scientists can form preconceived notions of how nature works and how diseases work, but we’re almost always wrong because nature is so complicated. But genetics is still one of the most powerful tools we can apply to understand how nature works.
“High-level scientific talent — the really creative people who are visionary — they understand how important this human genetic, proteomic, and transcriptomic data are for the future of our business. They want to work at a place like Amgen that is forging the lead in using these data to make medicines.”
The draw is not only in making medicines, but also in making them more valuable by reducing the uncertainty about which treatments may help a given patient. “With genetics and other human data, we’ll be able to predict, in a way we never could before, how a patient’s disease is going to evolve,” said Lenz. “That will allow us to distinguish between patients who need aggressive therapy and those for whom a watchful waiting approach is appropriate.”
Reese said that’s precisely the vison that’s driving Amgen. “In the long run, our goal is to provide the ultimate in personalized medicine—getting the right therapy in the right dose to the right patient at the right time. We have a chance to drive medicine in almost unimaginable directions, and I want to be part of that. I want Amgen to help lead it.”